
China Drying Network Recently, the Dumex milk powder incident has pushed food safety issues to the public. According to reports, the report pointed out that three Wahaha, Coca-Cola and Dumex companies in China purchased more than 200 tons of Fonterra whey protein powder that may have been contaminated with Clostridium botulinum. This incident caused the Food and Drug Administration and other departments to attach great importance.
According to the "Food Safety Law," its implementing regulations and other laws and regulations, and the "Notice of the General Office of the State Council Forwarding Food and Drug Administration and other departments on further strengthening the quality and safety of formula milk powder for infants and young children" (Guobanfa (2013) No. 57) Requirement: In order to tighten the licensing conditions for infant formula manufacturing enterprises, further improve the company's production equipment and facilities, control of raw and auxiliary materials, production process control, inspection and testing capabilities, personnel quality conditions, environmental condition control and independent research and development capabilities, etc. Infant and infant formula milk powder quality safety supervision, Food and Drug Administration General Organization of agriculture, food, medicine, dairy products industry and institutions of higher learning, scientific research institutions, experts, food safety supervision department personnel, set up a special drafting group, on the "production The rules for the review of the licensing conditions for infant formula milk powder (2010 version) were studied and amended to form the “Detailed Review Rules for the Production of Infant Formula Milk Powder Licensing Conditions (2013 version) (Draft for Comment)â€. The new detailed consultation draft seeks to comprehensively improve the production and management requirements of infant formula milk powder manufacturing enterprises with reference to pharmaceutical management. There are mainly: First, the implementation of HACCP and GMP management system; Second, the need for self-built milk control; Third, requires companies to have research and development capabilities; The fourth is to comprehensively improve the management requirements, especially raw materials procurement requirements; The requirements for comprehensively improving production conditions, such as improving the cleanliness of the production environment, and using purified water for production water, etc.
This review rule applies to enterprises that apply milk or goat milk and their processed products (whey powder, whey protein, skim milk powder, whole milk powder, etc.) as the main raw materials, and add appropriate amounts of vitamins, minerals and other auxiliary materials. Use the conditions required by laws, regulations, and standards to process infant formula milk powder, infant formula milk powder, infant formula milk powder for infants and young children (within 36 months of age), and review the company's production conditions and license production. Product inspection.
The application unit for infant formula milk powder is 1, and its category number is 0502. The production license product name must indicate infant formula milk powder (wet process, dry process), wet and dry composite process should be marked as wet process. Details on the specific types of infant formula milk powder, larger infant formula milk powder, and infant formula milk powder obtained from the production license are attached on the production license attached sheet.
Only the packaging site, process, and equipment, without complete production conditions, will not be subject to production license review; infant formula milk powder or base powder as the main raw material for the production of infant formula milk powder, there is no complete dry process, no production License review.
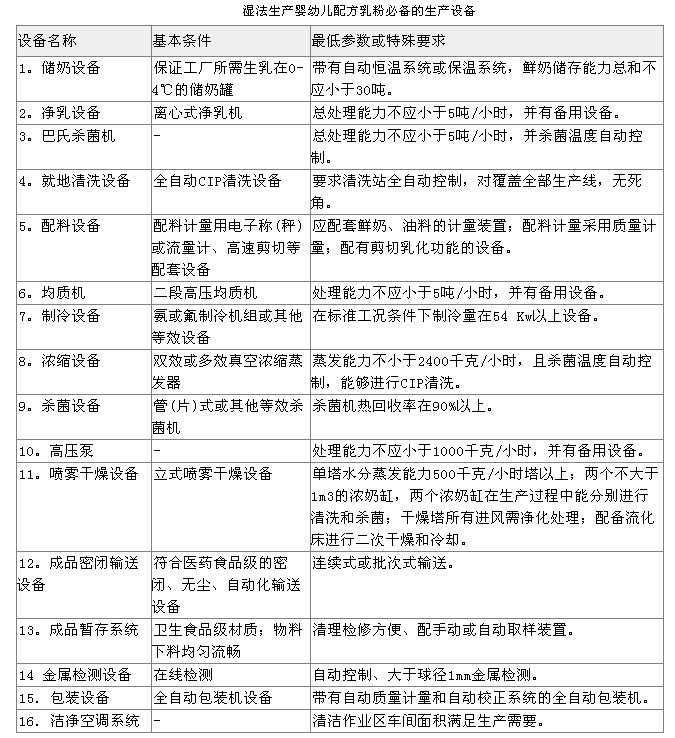
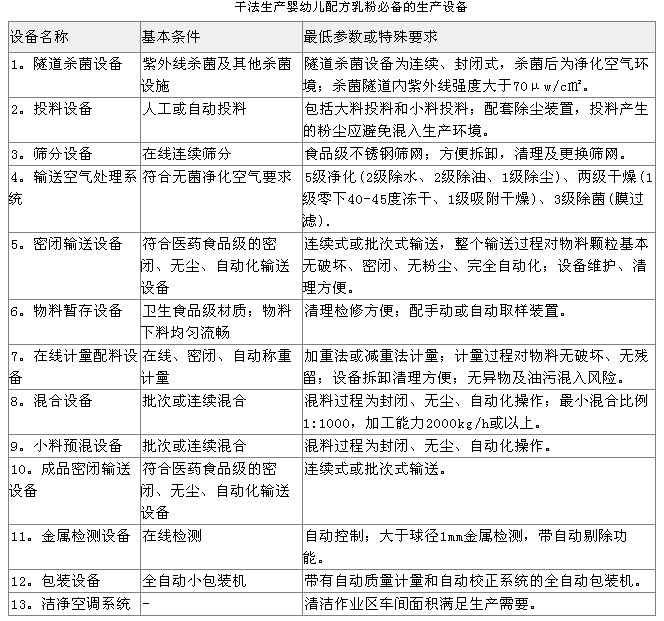
General requirements for production equipment in the rules
(1) Infant formula milk powder production enterprises shall have production facilities that are compatible with the design capabilities in the “Application for Food Production Permitâ€. (2) Equipment ledgers, manuals, resumes and files shall be kept in safekeeping; related procedures shall be established for the identification of the status of production and inspection equipment, designated personnel shall be responsible for management and recording, and the equipment and equipment status identification information shall be accurate. The management of the operating status of production equipment, common equipment, fixed pipeline facilities, measurement and inspection equipment, etc. shall be carried out. The definitions of various conditions and markings shall be defined, and the marking shall be checked and maintained on a regular basis. (3) All raw materials, process products, semi-manufactured containers and tools used in infant formula milk powder products must be made of Stainless Steel or other non-toxic inert materials. Bamboo and wooden tools shall not be used in the cleaning operation area; The containers must not be mixed with the containers containing the products and raw materials and should be clearly marked. (4) Wearing devices that are in direct contact with raw materials for production, such as glass thermometers, must have a safety jacket. (5) The air blown into the drying tower should be filtered, checked and replaced regularly to meet production requirements. Exhausted gas should be dedusted. (6) The equipment is well maintained and its performance and accuracy are in accordance with the production regulations. Equipment maintenance plans and maintenance records are complete. (7) The equipment needs to be verified after cleaning to ensure that the sanitary conditions of the equipment meet the production requirements. (8) The air purification treatment in the clean work area should adopt the three-stage filter of primary effect, medium effect, and high efficiency filter (sub-high efficiency Air Filter). The cleanliness of the cleanliness area shall be tested in both the static and dynamic conditions when the cleanliness of the cleanroom is confirmed at the factory confirmation stage or after installation of the process equipment or re-establishment of the clean work area due to other reasons (such as after large-scale maintenance in the clean work area); The medium and high-efficiency filters must detect planktonic bacteria, sedimentation bacteria, and surface microorganisms; in daily operation, the normal frequency of equipment detection and monitoring should be performed according to the following table.
Flexible Hose,Air Filter,Air Intake Pipe
Cooling Systerm Air Filter Co.,Ltd , http://www.chautopart.com